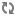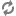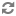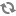The term extracorporeal membrane oxygenation (ECMO) was initially used to describe long-term extracorporeal support that focused on the function of oxygenation. Subsequently, in some patients, the emphasis shifted to carbon dioxide removal, and the term extracorporeal carbon dioxide removal was coined. Extracorporeal support was later used for postoperative support in patients following cardiac surgery. Other variations of its capabilities have been tested and used over the last few years, making it an important tool in the armamentarium of life and organ support measures for clinicians. With all of these uses for extracorporeal circuitry, a new term, extracorporeal life support (ECLS), has come into vogue to describe this technology.
The differences between ECMO and cardiopulmonary bypass are as follows:
-
ECMO is frequently instituted using only cervical cannulation, which can be performed under local anesthesia; standard cardiopulmonary bypass is usually instituted by transthoracic cannulation under general anesthesia
-
Unlike standard cardiopulmonary bypass, which is used for short-term support measured in hours, ECMO is used for longer-term support ranging from 3-10 days
-
The purpose of ECMO is to allow time for intrinsic recovery of the lungs and heart; a standard cardiopulmonary bypass provides support during various types of cardiac surgical procedures.
A diagram of extracorporeal membrane oxygenation is shown below.
 Extracorporeal membrane oxygenation (ECMO) system.
Extracorporeal membrane oxygenation (ECMO) system.
History of ECMO
In May 1953, Gibbon used artificial oxygenation and perfusion support for the first successful open heart operation.[1] In 1954, Lillehei developed the cross-circulation technique by using slightly anesthetized adult volunteers as live cardiopulmonary bypass apparatuses during the repair of certain congenital cardiac disorders.[2] In 1955, at the Mayo Clinic, Kirklin et al improved on Gibbon's device and successfully repaired an atrial septal defect.[3]
In 1965, Rashkind and coworkers were the first to use a bubble oxygenator as support in a neonate dying of respiratory failure.[4] In 1969, Dorson and colleagues reported the use of a membrane oxygenator for cardiopulmonary bypass in infants.[5]
In 1970, Baffes et al reported the successful use of extracorporeal membrane oxygenation as support in infants with congenital heart defects who were undergoing cardiac surgery.[6] In 1975, Bartlett et al were the first to successfully use ECMO in neonates with severe respiratory distress.[7]
Indications for ECMO
Patients with the following 2 major neonatal diagnoses require the use of ECMO:
-
Primary diagnoses associated with primary pulmonary hypertension of the newborn (PPHN), including idiopathic PPHN, meconium aspiration syndrome, respiratory distress syndrome, group B streptococcal sepsis, and asphyxia
Selection criteria for neonates include the following:
-
Gestational age of 34 weeks or more
-
Birth weight of 2000 g or higher
-
No significant coagulopathy or uncontrolled bleeding
-
No major intracranial hemorrhage (grade 1 intracranial hemorrhage)
-
Mechanical ventilation for 10-14 days or less
-
Reversible lung injury
-
No lethal malformations
-
No major untreatable cardiac malformation
-
Failure of maximal medical therapy
Failure to meet these criteria is a relative contraindication for ECMO.
Qualifying patient criteria for ECMO are applied only when the infant has reached maximal ventilatory support of 100% oxygen (fraction of inspired oxygen [FiO2] equals 1) with peak inspiratory pressures (PIP) often as high as 35 cm water. They include the alveolar-arterial gradient, the oxygenation index, and acute deterioration.
The alveolar-arterial (A-a) gradient of 600-624 mm Hg for 4-12 hours at sea level may be computed as follows (where 47=partial pressure of water vapor):
-
(A-a)(Diffusing capacity [D] of O2 equals atmospheric pressure - 47 - (PaCO2 + PaO2])/FiO2
The oxygenation index (OI) greater than 40 in 3 of 5 postductal gas determinations obtained 30-60 minutes apart may be computed as follows (where MAP is mean airway pressure):
-
OI = (MAP x FiO2 x 100)/PaO2
-
PaO2 = 35-50 mm Hg for 2-12 hours
Acute deterioration may be computed as follows:
-
PaO2 of 30-40 mm Hg or less for 2 hours
-
pH of 7.25 or less for 2 hours
-
Intractable hypotension
Pediatric ECMO is indicated or considered in the following situations:
-
Low cardiac output resulting from right, left, and biventricular failure following repair of congenital heart defect
-
Pulmonary vasoreactive crisis following repair of congenital heart defect leading to severe hypoxemia, low cardiac output, or both
-
Rarely, as a bridge to cardiac surgery in patients with serious end-organ damage resulting from profound low cardiac output related to congenital heart disease
-
As a bridge to cardiac transplant
-
Possibly, as a bridge to recovery in temporary cardiomyopathy secondary to renal failure, myocarditis, and burns.
Today, other indications or less strict selection of patients in certain institutions have made the use of this technology more diverse, not only in acute cardiac problems but also in primary pulmonary diseases.[8, 9, 10]
Contraindications to ECMO
The failure to meet selection criteria discussed in Indications for Extracorporeal Membrane Oxygenation, above, is a relative contraindication for ECMO.
Unlike the situation in neonates, when ECMO is considered in a pediatric patient, no clear set of inclusion or exclusion criteria exists. Evaluation of a pediatric patient for ECMO support is largely based on an assessment of the patient's condition and the institutional experience with pediatric ECMO.
Preparation
Equipment for ECMO
The extracorporeal membrane oxygenation (ECMO) apparatus consists of a blood pump with raceway tubing, a venous reservoir, a membrane oxygenator, and a countercurrent heat exchanger, as shown in the image below.
 Extracorporeal membrane oxygenation (ECMO) system.
Extracorporeal membrane oxygenation (ECMO) system.
The blood pump is either a simple roller pump (most common) or a constrained vortex centrifugal pump. The roller pump causes less hemolysis and is used for neonatal ECMO. The venous reservoir is used with the roller pump for neonatal ECMO. The oxygenator is responsible for exchanging both oxygen and carbon dioxide and is central to the successful performance of prolonged ECMO. Three types of commercial artificial lungs are available: bubble, membrane, and hollow-fiber devices. The heat exchanger warms the blood using a countercurrent mechanism. Blood is exposed to warm water that circulates within metal tubing.
Safety devices and monitors
Air bubble detectors can identify microscopic air bubbles in the arterialized blood and automatically turn off the blood pump.
Arterial line filters between the heat exchanger and the arterial cannula are used to trap air, thrombi, and other emboli.
Pressure monitors, which are placed before and after the oxygenator, measure the pressure of the circulating blood and are used to monitor for a dangerous rise in circuit pressure. This can occur with thrombosis of the oxygenator or occlusion of the tubing or cannulae. Pressure monitors are critical in preventing circuit disruption in the face of distal occlusion.
A continuous venous oxygen saturation monitor and temperature monitor are other important safety features.
Technique
Overview of ECMO Technique
The extracorporeal membrane oxygenation (ECMO) circuit is primed with the freshest blood available. The acid-base balance and blood gas of the primer are adjusted appropriately. Differences between venoarterial ECMO and venovenous ECMO are presented below (also see the Table).
Venoarterial bypass
The standard ECMO procedure used in most neonatal ICUs is venoarterial bypass. In this situation, a cannula is placed through the right jugular vein into the right atrium. Blood is drained to a venous reservoir located 3-4 feet below heart level. The blood is actively pumped by a roller pump through the oxygenator, where gas exchange occurs via countercurrent flow of blood and gas. Next, the blood is warmed to body temperature by the heat exchanger before returning to the patient through a cannula placed through the right carotid artery into the aortic arch. Systemic anticoagulation therapy with heparin is administered throughout the bypass circuit, with frequent monitoring of activated clotting time (ACT), which should be maintained at 180-240 seconds.
Venovenous bypass
In venovenous bypass, a double-lumen cannula is placed through the right jugular vein into the right atrium. Desaturated blood is withdrawn from the right atrium through the outer fenestrated venous catheter wall, and oxygenated blood is returned through the inner lumen of the catheter and is angled to direct blood across the tricuspid valve.
Table. Differences Between Venoarterial and Venovenous Extracorporeal Membrane Oxygenation (Open Table in a new window)
| Venoarterial ECMO | Venovenous ECMO |
| Higher PaO2 is achieved. | Lower PaO2 is achieved. |
| Lower perfusion rates are needed. | Higher perfusion rates are needed. |
| Bypasses pulmonary circulation | Maintains pulmonary blood flow |
| Decreases pulmonary artery pressures | Elevates mixed venous PO2 |
| Provides cardiac support to assist systemic circulation | Does not provide cardiac support to assist systemic circulation |
| Requires arterial cannulation | Requires only venous cannulation |
In certain patients with cardiac or respiratory failure who have recently undergone cardiac surgery, transthoracic cannulation of the right atrial appendage and the aortic arch can be used as an alternative to neck cannulation. Transthoracic cannulation allows left heart decompression by cannulation of the left atrium. This is useful in patients with primary left heart failure.
Pulmonary System Management
ECMO is used temporarily while awaiting pulmonary recovery. In the classic use of neonatal ECMO, the typical ventilator settings are FiO2 of 21-30%, peak inspiratory pressure (PIP) of 15-25 cm H2 O, a positive end-expiratory pressure (PEEP) of 3-5 cm H2 O, and intermittent mechanical ventilation (IMV) of 10-20 breaths per minute. In some centers, a high PEEP of 12-14 cm water has been used to avoid atelectasis; this has been found to shorten the bypass time in infants. Pulmonary hygiene is strict and requires frequent positional changes, endotracheal suctioning every 4 hours depending on secretions, and a daily chest radiograph.
Cardiovascular System Management
Systemic perfusion and intravascular volume should be maintained. Volume status can be assessed clinically by urine output and physical signs of perfusion and by measuring the central venous pressure and the mean arterial blood pressure. Native cardiac output can be enhanced with inotropic agents. Echocardiography should be performed to exclude any major congenital heart anomaly that may require immediate intervention other than ECMO (eg, obstructed total anomalous pulmonary venous connection).
CNS Management
Central nervous system complications are the most serious and are primarily related to the degree of hypoxia and acidosis. Avoiding paralytic agents and performing regular neurologic examinations are recommended. If feasible, head ultrasonography should be performed before beginning ECMO in a neonate. Reevaluation with serial head ultrasonography may be needed on a daily basis, especially after any major event. In patients with seizures or suspected seizures, aggressive treatment is recommended (eg, phenobarbital).
Renal System Management
During the first 24-48 hours on ECMO, oliguria and acute tubular necrosis associated with capillary leak and intravascular volume depletion are common because ECMO triggers an acute inflammatory-like reaction. The diuretic phase, which usually begins within 48 hours, is often one of the earliest signs of recovery. If oliguria persists for 48-72 hours, diuretics are often Bắt buộc to reduce edema. When renal failure does not improve, hemofiltration or hemodialysis filters may be added to the circuit.
Hematologic Considerations
To optimize oxygen delivery, the patient's hemoglobin should be maintained at 12-15 g/dL using packed RBCs (pRBCs). As a result of platelet consumption during ECMO, platelet transfusions are Bắt buộc to maintain platelet counts above 100,000/mcL. Activated clotting time (ACT) should be maintained at 180-240 seconds to avoid bleeding complications.
Infection Control
Strict aseptic precautions are Bắt buộc. The presence of infection is monitored by obtaining cultures from the circuit at least once a week. Based on institutional experience, the protocol frequency may vary. Other appropriate cultures (eg, fungal and viral) should be obtained as needed.
Fluids, Electrolytes, and Nutrition
Patients on ECMO require close monitoring of fluids and electrolytes. The high-energy requirements should be met using hyperalimentation techniques. The patient's weight increases in the first 1-3 days on ECMO because of fluid retention.
Post-Procedure
Mechanical Complications
Clots in the circuit are the most common mechanical complication (19%). Major clots can cause oxygenator failure, consumption coagulopathy, and pulmonary or systemic emboli. More recently, heparin-coated extracorporeal membrane oxygenation (ECMO) systems have been used to decrease the frequency of this complication.
Cannula placement can cause damage to the internal jugular vein, which causes massive mediastinal bleeding. Dissection of the carotid arterial intima can lead to lethal aortic dissection.
Air in the circuit can range from a few bubbles to a complete venous air lock. This air can originate in the dislodgement of the venous cannula, a small tear in the membrane, or high partial pressure of oxygen in the blood. A large bolus of air can be fatal.
Oxygenator failure is defined either as decreased oxygen or carbon dioxide transfer or as the presence of consumptive coagulopathy. A failing membrane should be replaced immediately.
Cracks in the connectors and tube rupture have become less serious problems since the introduction of Tygon raceway tubing.
Pump malfunction may be a manifestation of inadequate venous return to the pump; heat exchanger malfunction can cause severe hypothermia.
Failure of the entire circuit, including the oxygen source and oxygen blenders, may occur, as may failure of circuit-monitoring equipment. In cases of circuit failure, immediately clamp the venous line, open the bridge, and clamp the arterial line to remove the patient from the ECMO. Because the patient is ventilator dependent, immediately bag the patient with 100% oxygen (FiO2 =1) or shift the patient back to pre-ECMO ventilator settings.
Medical Complications
Neurologic complications include seizures. Intracranial bleeds and infarction may be due to ligation of the carotid artery and internal jugular vein, systemic heparinization, thrombocytopenia, coagulopathies, or systolic hypertension.
Hemorrhagic complications include hemorrhages and a decreased platelet count. Hemolysis and consumption coagulopathy may occur. Hemorrhage at the surgical site, at the cannula site, or into the site of a previous invasive procedure is a frequent complication because of systemic heparinization. Intrathoracic, abdominal, or retroperitoneal hemorrhage may also occur. Decreases in the platelet count occur because of decreased production, increased consumption, sequestration, or dilution.
Cardiac complications include myocardial stun, which is defined as a decrease in the left ventricular shortening fraction by more than 25% with initiation of ECMO that returns to normal after 48 hours of ECMO. In addition, hypertension is a dangerous complication because of the risk of hemorrhage and stroke. Arrhythmia may occur as a result of hypoxia and electrolyte imbalance. Symptomatic patent ductus arteriosus may occur, as well as pericardial tamponade may occur.
Pneumothorax is a potential pulmonary complication, along with pulmonary hemorrhage.
Oliguria is a commonly observed renal complication during the early part of ECMO; acute tubular necrosis is observed in some patients and may require hemofiltration and dialysis.
GI tract complications include hemorrhage, which may occur as a result of stress, ischemia, or bleeding tendencies. Direct hyperbilirubinemia and biliary calculi may occur secondary to prolonged fasting and total parenteral nutrition (TPN), hemolysis, and diuretics.
Complications may also result from infection and sepsis, because the ECMO circuit represents a large intravascular foreign body, and frequent manipulation increases the risk of sepsis.
Metabolic complications include the following:
-
Hyperglycemia or hypoglycemia
ECMO may alter serum concentration of drugs due to increased volume of distribution. Caution is warranted when narrow therapeutic drugs are administered, and dose alterations may be necessary.
Acute cardiorespiratory decompensation may result because of the following:
-
Pericardial tamponade (from blood or air)
-
Tension pneumothorax or hemothorax
-
Respiratory failure
-
Myocardial ischemia
-
Electrolyte imbalance
-
Massive hemorrhage (especially intracranial hemorrhage)
-
Drug effects
-
Overwhelming sepsis
Weaning or Trial Period Without ECMO
In patients with a principal pre-ECMO diagnosis of respiratory failure, a trial period without ECMO is scheduled if (1) the patient demonstrates adequate gas exchange and is on reasonable ventilatory settings and (2) the patient tolerates a pump flow of 10-20 mL/kg/min with the minimum of 200 mL/min. Variable weaning times are followed in patients on ECMO. In addition, the duration of treatment varies.
Mortality and Morbidity Associated with ECMO
Mortality statistics of ECMO-treated patients have remained stable over the past decade. Predictors of death include the following:
-
Patients with a primary diagnosis of congenital diaphragmatic hernia (CDH) and total anomalous pulmonary venous returns (TAPVR) have a mortality rate of 50%
-
Approximately 50% of reported deaths are due to severe bleeding complications
-
The mortality rate is high in infants with a birth weight less than 2000 g
Infants who survive following ECMO have a higher rate of rehospitalization for nonpulmonary and surgical conditions. Approximately 15% of infants still require oxygen at 28 days after ECMO. These children have a higher rate of rehospitalization for pulmonary indications, particularly in the first 6 months after ECMO, and have a slightly higher prevalence of bronchial asthma.
Difficulty in establishing full oral feeding is common after ECMO decannulation; feeding difficulty is reported in as many as one third of babies, even in the presence of normal suck and swallow reflexes. Somatic growth is normal in infants who survive following ECMO, and poor growth should be evaluated for another underlying cause.
Both clinical and electroencephalographic seizure activity is reported in 20-70% of neonates while on ECMO. Epilepsy is reported in 2% of patients at age 5 years.
Sensorineural disabilities
The rate of sensorineural disabilities in infants who survive following ECMO averages 6% (range, 2-18%); developmental delay occurs in 9% (range, 0-21%). Abnormal brainstem auditory-evoked response (BAER) with mild-to-moderate threshold elevation is seen in 25% of children following ECMO at discharge. This condition usually resolves. Sensorineural hearing loss is documented after age 1 year in 9% (range, 4-21%) of children following ECMO.
Routine ophthalmic examinations during ECMO are not recommended because studies in term babies have not shown any occurrence of retinopathy. In the rare neonate with birth weight less than 2 kg in whom ECMO is used, ophthalmic examination is Bắt buộc prior to discharge. Some degree of cortical visual impairment has been seen after posterior brain injury. However, in the long term, visual function has been shown to improve.
Psychosocial morbidity
Psychosocial morbidity includes the increased frequency of social problems, academic difficulties at school age, and higher rates of attention deficit disorder in children who received ECMO. In addition, the ECMO procedure is dramatic and highly invasive, and families can feel isolated if no other patients are on ECMO in the same institution.
At age 1 year, the stress level of the mother of an infant previously on ECMO is the same as the stress level in the family of a preterm infant. By age 5 years, the family stress level is the same as that of the family of a healthy child in whom ECMO was not used.
Neuromotor deficits
Neuromotor deficits range from mild hypotonia to gross motor delay and spastic quadriparesis. Glass and colleagues compared the neurodevelopmental outcome of 103 neonates following ECMO and 37 neonates without ECMO at 5 age years[11] ; the mean full-scale, verbal, and performance intelligence quotient (IQ) scores of children who received ECMO treatment were within the normal range. As a group, however, the scores were significantly lower than in children who had not had ECMO (96 vs 115). Major disability, which was defined as mental disability, motor disability, sensorineural impairment, or seizure disorder, was present in 17 of children in whom ECMO had been used.
Other studies have proven that the neurodevelopmental outcome of the ECMO cohort is comparable to other high-risk neonatal groups and similar to neonates with the same condition managed conventionally.
Medications and Medical Devices
Medication Summary
Aminocaproic acid may be Bắt buộc to reduce bleeding during surgery. Only minimal sedation with fentanyl, midazolam, or morphine is Bắt buộc after stabilization. Doses of most inotropic medications, such as dopamine, dobutamine, and epinephrine, can usually be decreased once the patient is on extracorporeal membrane oxygenation (ECMO).
Diuretics, such as furosemide (Lasix) and chlorothiazide (Diuril), may be Bắt buộc for mobilization of tissue fluids. Antacids and H2 antagonists are usually administered for GI tract bleeding. Phenobarbital can be used if the patient has seizures.
Antibiotics, such as ampicillin and cefotaxime, are used initially in the typical septicemic dosages; dosage modification may be needed, depending on the pathogen and sensitivity.
The duration of treatment will vary based on the initial diagnosis and the daily evaluation of the patient. It should be remembered that the longer the patient remains in this therapy, the more complications the patient may experience.